Understanding the Regulatory Framework
The Legal Foundation: DSHEA and Beyond
Supplement labeling regulations stem from several key pieces of legislation that work together to ensure consumer safety and informed decision-making:
The Nutrition Labeling and Education Act of 1990
This foundational law amended the Federal Food, Drug, and Cosmetic Act (FD&C Act) by requiring that most foods, including dietary supplements, bear nutrition labeling. This legislation established the groundwork for standardized nutritional information disclosure.
The Dietary Supplement Health and Education Act of 1994 (DSHEA)
DSHEA specifically addressed dietary supplements by:
- Defining what constitutes a dietary supplement
- Establishing specific labeling requirements for supplements
- Creating the framework for structure/function claims
- Requiring manufacturers to ensure safety and proper labeling before marketing
Current Regulatory Authority
Under DSHEA, manufacturers and distributors are prohibited from marketing products that are adulterated or misbranded. This means companies are responsible for evaluating the safety and labeling of their products before marketing to ensure compliance with all federal requirements.
Key Regulatory Agencies
FDA's Center for Food Safety and Applied Nutrition (CFSAN)
- Primary oversight responsibility for dietary supplements
- Inspects manufacturing facilities and reviews labeling compliance
- Investigates consumer complaints and adverse event reports
- Monitors the supplement marketplace for violations
Office of Dietary Supplement Programs (ODSP)
Established in 2015, ODSP specifically focuses on:
- Monitoring safety and labeling claims of dietary supplements
- Reviewing new dietary ingredient (NDI) notifications
- Coordinating enforcement actions for non-compliant products
Five Required Label Elements
FDA regulations mandate that all dietary supplement containers and packages include five essential label statements. Missing any of these elements can result in your product being considered misbranded.
1. Statement of Identity (Product Name)
Requirements:
- Must clearly identify the product as a dietary supplement
- Should be prominent and easily readable
- Cannot be misleading about the product’s contents or benefits
- Must use the term “dietary supplement” or specify the type (e.g., “vitamin supplement,” “herbal supplement”)
Examples of Compliant Statements:
- “Vitamin D3 Dietary Supplement”
- “Omega-3 Fish Oil Supplement”
- “Multivitamin and Mineral Supplement”
2. Net Quantity of Contents Statement
Requirements:
- Must accurately declare the amount of product in the container
- Should be displayed prominently on the principal display panel (PDP)
- Must use appropriate units of measurement
- Cannot be misleading about the actual quantity
Common Formats:
- “60 Capsules”
- “120 Tablets”
- “16 fl oz (473 mL)”
- “90 Servings”
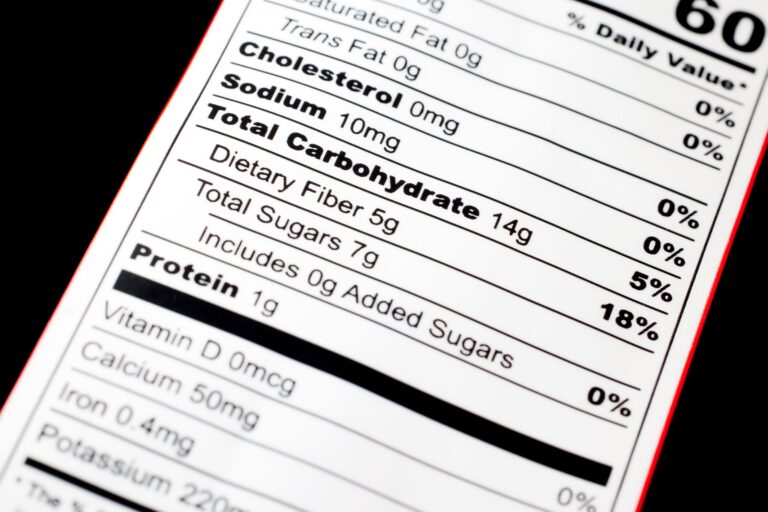
3. Nutrition Labeling (Supplement Facts Panel)
The Supplement Facts panel is the heart of supplement labeling, providing detailed nutritional information in a standardized format that consumers can easily understand and compare across products.
4. Ingredient List
Requirements:
- Must list all ingredients in descending order by weight
- Include both active and inactive ingredients
- Use proper ingredient names as specified in FDA databases
- Declare potential allergens according to major food allergen labeling requirements
Supplement Facts Panel Requirements
Panel Format and Structure
The Supplement Facts panel must follow specific formatting requirements to ensure consistency and readability across all supplement products.
Requirements:
- Title: “Supplement Facts” at the top of the panel
- Serving Size: Maximum amount recommended per eating occasion
- Servings Per Container: Total number of servings (if different from net quantity)
- Amount Per Serving: Quantity of each ingredient per serving
- Percent Daily Value (%DV): Where established daily values exist
Displaying Dietary Ingredients
Ingredients with Established Daily Values:
- Must show amount per serving and %DV
- Include vitamins, minerals, and other nutrients with RDIs or DRVs
- Use standardized units of measurement
Ingredients without Established Daily Values:
- Show amount per serving with an asterisk (*)
- Include footnote: “Daily Value not established”
- Common for proprietary blends and novel ingredients
Proprietary Blends
Special Requirements:
- Must be identified as “Proprietary Blend” or similar descriptive term
- Show total weight of the blend
- List individual ingredients in descending order by weight
- Cannot show individual amounts of ingredients within the blend
- Still must comply with all other labeling requirements
Types of Claims and Legal Requirements
Understanding the different types of claims you can make on supplement labels is crucial for compliance. Each claim type has specific requirements and limitations.
Structure/Function Claims
What They Are:
Structure/function claims describe how a supplement affects normal bodily functions or structures without referencing disease.
Compliant Examples:
- “Supports immune system function”
- “Helps maintain healthy bones”
- “Promotes cardiovascular health”
- “Supports energy metabolism”
Requirements:
- Must submit notification to FDA within 30 days of first marketing
- Must include required disclaimer on label
- Must have substantiation that claims are truthful and not misleading
- Cannot imply disease treatment, diagnosis, cure, or prevention
Required Disclaimer:
“This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.”
Nutrient Content Claims
What They Are:
Claims that characterize the level of a nutrient in a supplement relative to established standards.
Common Examples:
- “High in Vitamin C”
- “Good source of Iron”
- “Low sodium”
- “Antioxidant”
Requirements:
- Must meet specific quantitative criteria established by FDA
- Use only FDA-approved terminology
- Cannot be misleading about nutrient levels
- May require additional qualifying statements
Health Claims
What They Are:
Statements that describe a relationship between a dietary ingredient and reduced risk of disease or health condition.
Requirements:
- Must receive FDA pre-approval through petition process
- Requires “significant scientific agreement” standard
- Limited number of authorized health claims for supplements
- Must use specific, FDA-approved language
Examples of Authorized Health Claims:
- Calcium and reduced risk of osteoporosis
- Folic acid and reduced risk of neural tube defects
- Omega-3 fatty acids and reduced risk of coronary heart disease
Qualified Health Claims
What They Are:
Health claims that don’t meet the “significant scientific agreement” standard but have some credible scientific evidence.
Requirements:
- Must include qualifying language about the level of scientific evidence
- Require FDA review and approval of specific claim language
- Less common for dietary supplements than conventional foods
FDA vs FTC Jurisdiction: Understanding Dual Oversight
FDA Responsibilities (Labeling)
Primary Jurisdiction:
- Product labels and packaging
- Inserts and materials at point of sale
- Structure/function claim notifications
- Good Manufacturing Practice (GMP) compliance
Enforcement Actions:
- Warning letters for labeling violations
- Product recalls for safety issues
- Facility inspections and compliance monitoring
- Injunctions against non-compliant manufacturers
Primary Jurisdiction:
- All forms of advertising (TV, radio, print, digital)
- Website marketing claims
- Social media promotional content
- Third-party endorsements and testimonials
Key Requirements:
- Claims must be substantiated with “competent and reliable scientific evidence”
- Cannot make false or misleading statements
- Must disclose material connections with endorsers
- Applies same standards regardless of product type
Overlapping Areas and Coordination
Shared Jurisdiction:
Both agencies can take action against marketers making deceptive labeling claims. They coordinate enforcement efforts through a Memorandum of Understanding (FDA-FTC Liaison Agreement).
Important Distinctions:
- FDA: Requires structure/function claim notifications but doesn’t pre-approve claims
- FTC: No notification requirement but requires prior substantiation
- FDA: Distinguishes between claim types and product categories
- FTC: Applies uniform standards regardless of claim or product type
Common Labeling Violations and How to Avoid Them
Misbranding Through Disease Claims
The Problem:
Making unauthorized disease claims turns your supplement into an unapproved drug, violating FDA regulations.
Common Violations:
- “Cures diabetes”
- “Treats arthritis pain”
- “Prevents heart disease”
- “Fights cancer”
How to Avoid:
- Focus on structure/function language
- Avoid disease-specific terminology
- Don’t reference specific medical conditions
- Use words like “supports,” “maintains,” or “promotes”
Inadequate Substantiation
The Problem:
Making claims without sufficient scientific evidence to support them.
Common Issues:
- Relying solely on animal studies
- Using outdated or irrelevant research
- Making claims beyond what the evidence supports
- Failing to maintain substantiation files
Best Practices:
- Compile comprehensive substantiation dossiers
- Use human clinical trials when available
- Consider hiring expert review of evidence
- Regularly update substantiation as new research emerges
Allergen Labeling Failures
Major Food Allergens That Must Be Declared:
Milk, eggs, fish, shellfish, tree nuts, peanuts, wheat, soybeans, sesame
Requirements:
- Must be declared even if present in small amounts
- Include sub-ingredients and processing aids
- Use clear, prominent allergen statements
- Consider “may contain” statements for cross-contamination risks
2025 Labeling Updates and Changes
Recent FDA Developments
Updated Allergen Requirements:
Sesame was added as the 9th major food allergen in 2021, requiring declaration on all supplement labels by January 1, 2023.
Front-of-Package Nutrition Labeling:
FDA proposed new front-of-package nutrition labeling requirements in January 2025, though dietary supplements are currently proposed to be exempt.
Updated "Healthy" Nutrient Content Claim:
FDA finalized new definitions for “healthy” claims in late 2024, which now include dietary supplements that meet applicable criteria.
Compliance Dates and Deadlines
Uniform Compliance Date:
FDA established a uniform compliance date for food labeling regulations published from January 1, 2025, to December 31, 2026, helping manufacturers manage multiple regulatory changes.
Planning for Changes:
- Monitor FDA guidance documents for updates
- Plan label revisions well in advance of compliance dates
- Consider impact on inventory and manufacturing schedules
- Work with regulatory experts to ensure timely compliance
Best Practices for Compliance
Establishing a Labeling Review Process
Internal Review Checklist:
- Verify all five required label elements are present
- Check Supplement Facts panel formatting and accuracy
- Review all claims for compliance with FDA guidelines
- Confirm ingredient list accuracy and allergen declarations
- Validate contact information and regulatory statements
External Expert Review:
- Engage regulatory consultants for complex products
- Have lawyers review claim substantiation
- Consider third-party label audits
- Get scientific expert opinions on health claims
Documentation and Record-Keeping
Essential Records to Maintain:
- Complete substantiation files for all claims
- Certificates of analysis for all ingredients
- Label approval documentation
- Structure/function claim notifications to FDA
- Adverse event reports and responses
Retention Requirements:
- Keep all records for at least three years
- Maintain electronic and physical copies
- Organize files for easy FDA inspection access
- Update records as formulations or labels change
Quality Control Systems
Label Management Software:
Consider using specialized software for:
- Version control and approval workflows
- Automated compliance checking
- Multi-location label coordination
- Regulatory update notifications
Supplier Verification:
- Require certificates of analysis from ingredient suppliers
- Verify organic or other special certifications
- Confirm allergen status and cross-contamination controls
- Maintain updated supplier qualification files
Working with Professional Label Designers
When to Hire Experts
Complex Products:
- Products with multiple ingredients or complex formulations
- International products requiring multi-country compliance
- Products making health or structure/function claims
- First-time supplement manufacturers
Regulatory Changes:
- When new regulations take effect
- After FDA warning letters or regulatory issues
- Before launching in new markets or channels
- During major product reformulations
What to Expect from Professional Services
Comprehensive Label Review:
- Regulatory compliance verification
- Claim substantiation assessment
- Competitive analysis and positioning
- Consumer testing and readability analysis
Ongoing Support:
- Regulatory update monitoring
- Label revision management
- FDA communication assistance
- Training for internal teams
At Health Genesis, we understand that compliant supplement labeling is just the beginning. Our comprehensive label design and regulatory services include:
Full Regulatory Compliance:
- Complete FDA and FTC compliance review
- Structure/function claim development and notification
- Supplement Facts panel creation and verification
- Allergen and ingredient declaration accuracy
Professional Graphic Design:
- Eye-catching, consumer-friendly designs
- Brand-consistent visual elements
- Shelf appeal optimization
- Multiple format and size options
Expert Consultation:
- Regulatory strategy development
- Claim substantiation guidance
- Market positioning recommendations
- Ongoing compliance monitoring
Comprehensive Services:
- Label printing and production coordination
- Inventory management and logistics
- Regulatory filing assistance
- Quality assurance and testing
Ready to ensure your supplement labels meet all FDA requirements while maximizing market appeal? Contact our expert design team today for a comprehensive label review and consultation.
Frequently Asked Questions
Do I need FDA approval for my supplement labels?
No, FDA does not pre-approve dietary supplement labels. However, you must ensure your labels comply with all applicable regulations before marketing. FDA reviews labels after products enter the marketplace and can take enforcement action against non-compliant products.
How long do I have to notify FDA about structure/function claims?
You must submit a notification to FDA within 30 days of first marketing a product that makes structure/function claims. The notification must include the exact text of the claim as it appears on the label.
Can I make health claims on my supplement labels?
Only pre-approved health claims with significant scientific agreement can be used. Most health claims available for supplements are limited to specific nutrients like calcium, folic acid, and omega-3 fatty acids. Unauthorized health claims can result in your product being considered an unapproved drug.
What happens if my label is incorrect or non-compliant?
Non-compliant labels can result in:
- FDA warning letters
- Product recalls
- Facility inspections
- Injunctions and legal action
- FTC enforcement for advertising violations
- Loss of consumer trust and brand damage
How do I know if my claims are properly substantiated?
Claims must be supported by “competent and reliable scientific evidence.” This typically includes:
- Well-designed human clinical trials
- Peer-reviewed published research
- Multiple studies supporting the same conclusion
- Evidence directly relevant to your specific product and claims
Consider having your substantiation reviewed by scientific and regulatory experts.
What's the difference between "organic" and other ingredient designations on labels?
Organic ingredients must meet USDA National Organic Program standards and be certified by an accredited certifying agent. Other terms like “natural” are largely unregulated and can be misleading. Only use organic designations for properly certified ingredients.
Can I use the same label for products sold online and in stores?
Yes, the same labeling requirements apply regardless of where products are sold. However, online marketing materials and website claims fall under FTC jurisdiction and must also comply with advertising substantiation requirements.
How often should I review and update my supplement labels?
Review labels:
- Whenever regulations change
- Before reformulating products
- At least annually as part of quality system reviews
- After receiving any regulatory feedback or consumer complaints
- When launching in new markets or distribution channels
What should I do if FDA or FTC contacts me about my labels?
- Respond promptly and professionally
- Gather all relevant documentation
- Consider engaging regulatory attorneys or consultants
- Don’t admit fault or make statements without legal review
- Develop a corrective action plan if violations are identified
How can I stay updated on changing supplement labeling regulations?
- Subscribe to FDA and FTC guidance updates
- Join industry associations like CRN or AHPA
- Work with regulatory consultants
- Attend industry conferences and webinars
- Monitor FDA warning letters and enforcement actions